
Approach

Approach
At Bolt, we are leveraging the immune system for a better way to treat cancer. We are open to partnering the programs featured on this page, and entering into collaborations built around our Boltbody™ ISAC technology.
Partnering inquiries should be directed to partnering@boltbio.com.
Proprietary Bolt Programs Available for Partnering
BDC-3042: P2-ready Dectin-2 Agonist mAb
Colorectal Cancer, Non-small Cell Lung Cancer
Preclinical CEA ISAC
Colorectal Cancer, Non-small Cell Lung Cancer
Preclinical PD-L1 ISAC
Solid Tumor Resistant to Checkpoint Inhibitors
We have existing collaborations based on our Boltbody™ ISAC platform with Genmab and Toray. The Genmab collaboration funds up to 3 Boltbody ISACs through early clinical development, and the Toray collaboration funds a Boltbody ISAC targeting Caprin-1 through early clinical development.

Boltbody™ ISAC Platform
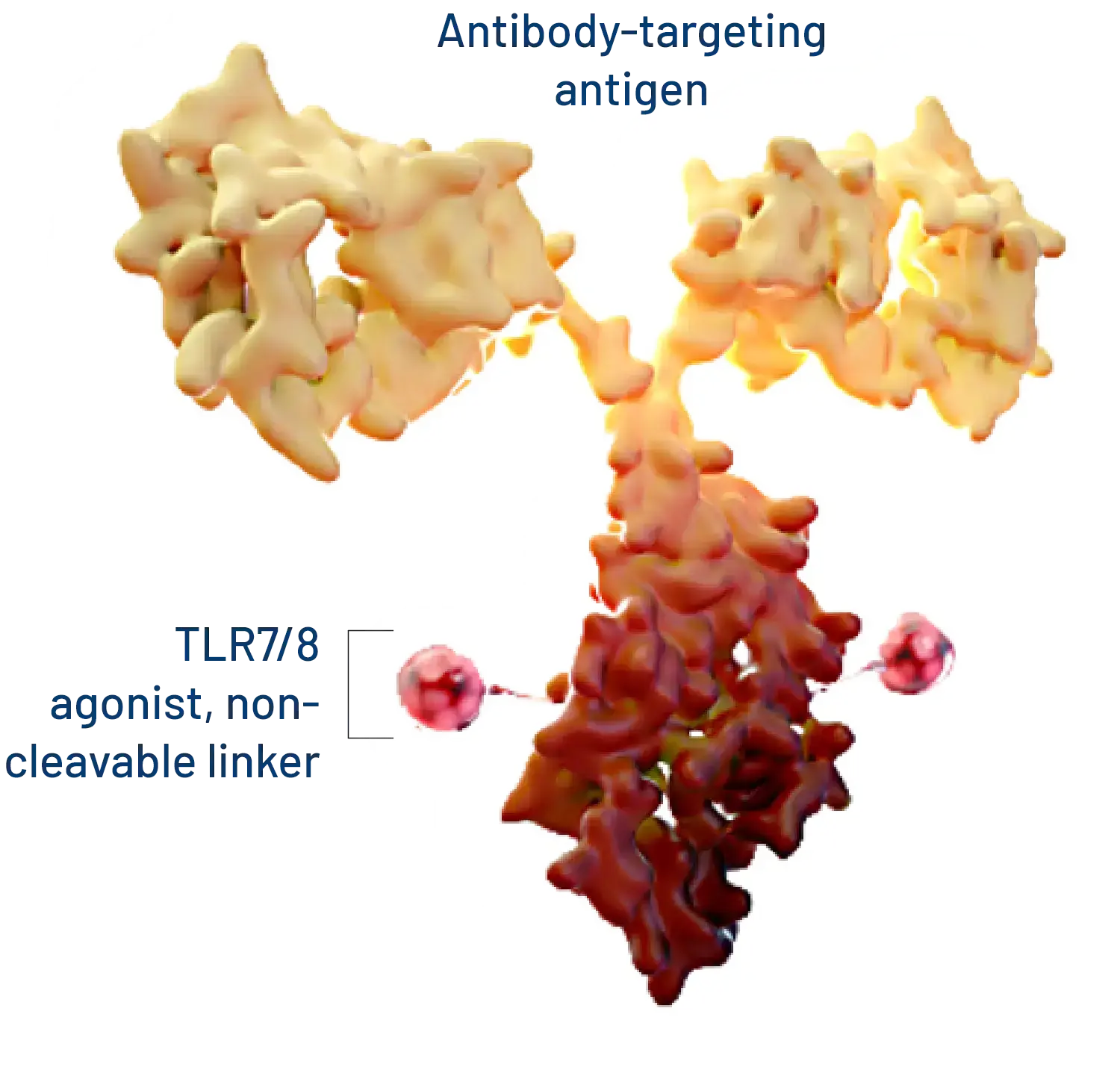
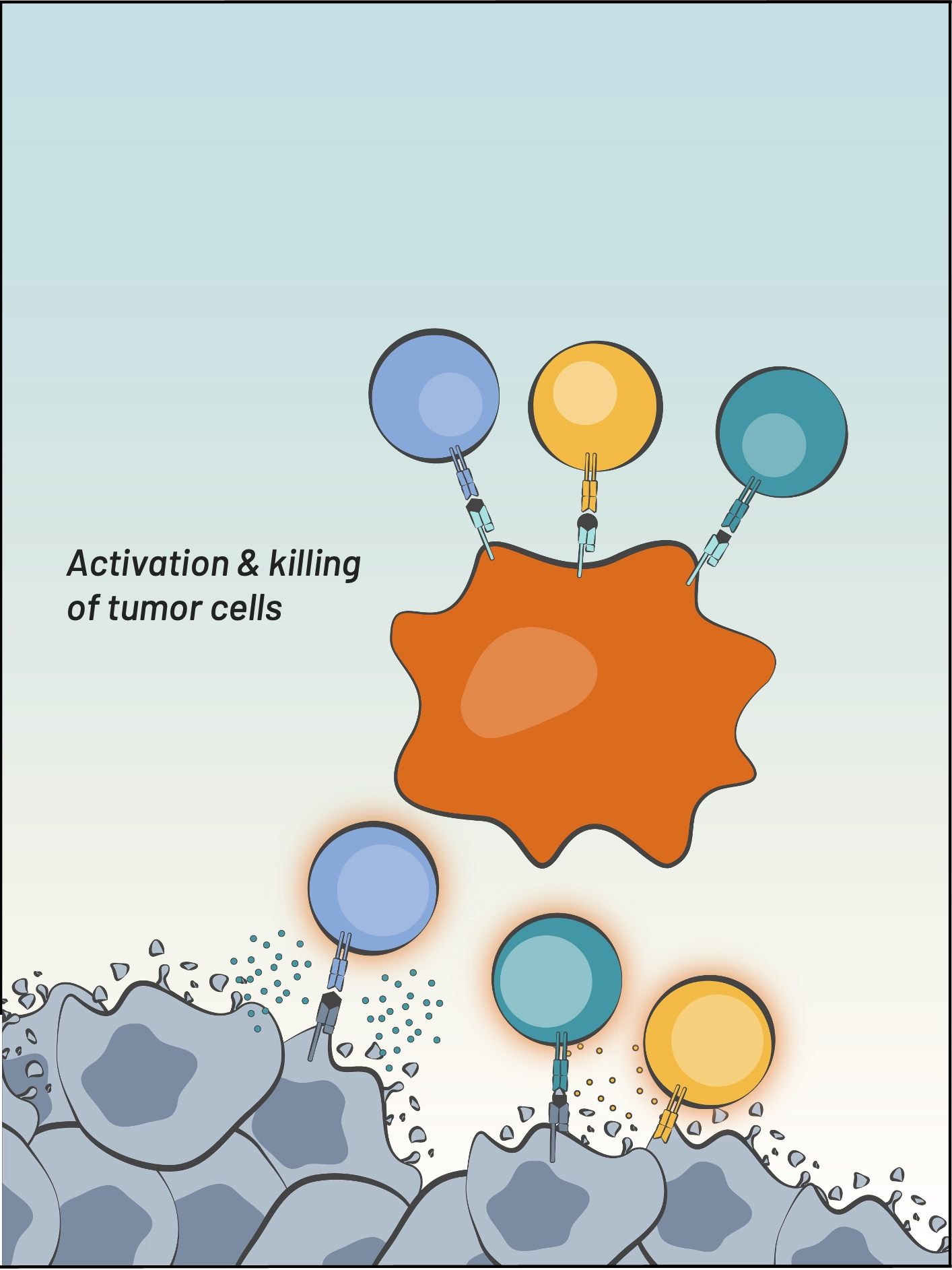
Our Immune-Stimulating Antibody Conjugate (ISAC) platform technology enables tumor-specific activation of the innate immune system which generates a durable anti-cancer adaptive immune response. ISACs use the precision of antibody targeting to harness the power of the innate immune system, which reprograms the tumor microenvironment and invokes a new anti-tumor immune response that can spread to the adaptive immune system.
KEY FEATURES
Only ISAC platform with emerging clinical validation
Single-agent activity demonstrated preclinically with many different tumor targets
Ability to address difficult-to-treat solid tumors, including those refractory to current treatments
Pipeline engine that is widely applicable

KEY FEATURES
Only ISAC platform with emerging clinical validation
Single-agent activity demonstrated preclinically with many different tumor targets
Ability to address difficult-to-treat solid tumors, including those refractory to current treatments
Pipeline engine that is widely applicable
Our Boltbody ISACs are delivered systemically but act locally in the tumor microenvironment by triggering a localized anti-tumor immune cascade. This process enables the patients’ own immune system to determine which are the relevant T cells to mobilize for tumor destruction and subsequent immunosurveillance, so an off-the shelf Boltbody immunotherapeutic can deliver a personalized therapeutic outcome. We believe that the development of systemic immunological memory will generate durable therapeutic responses for patients with cancer.
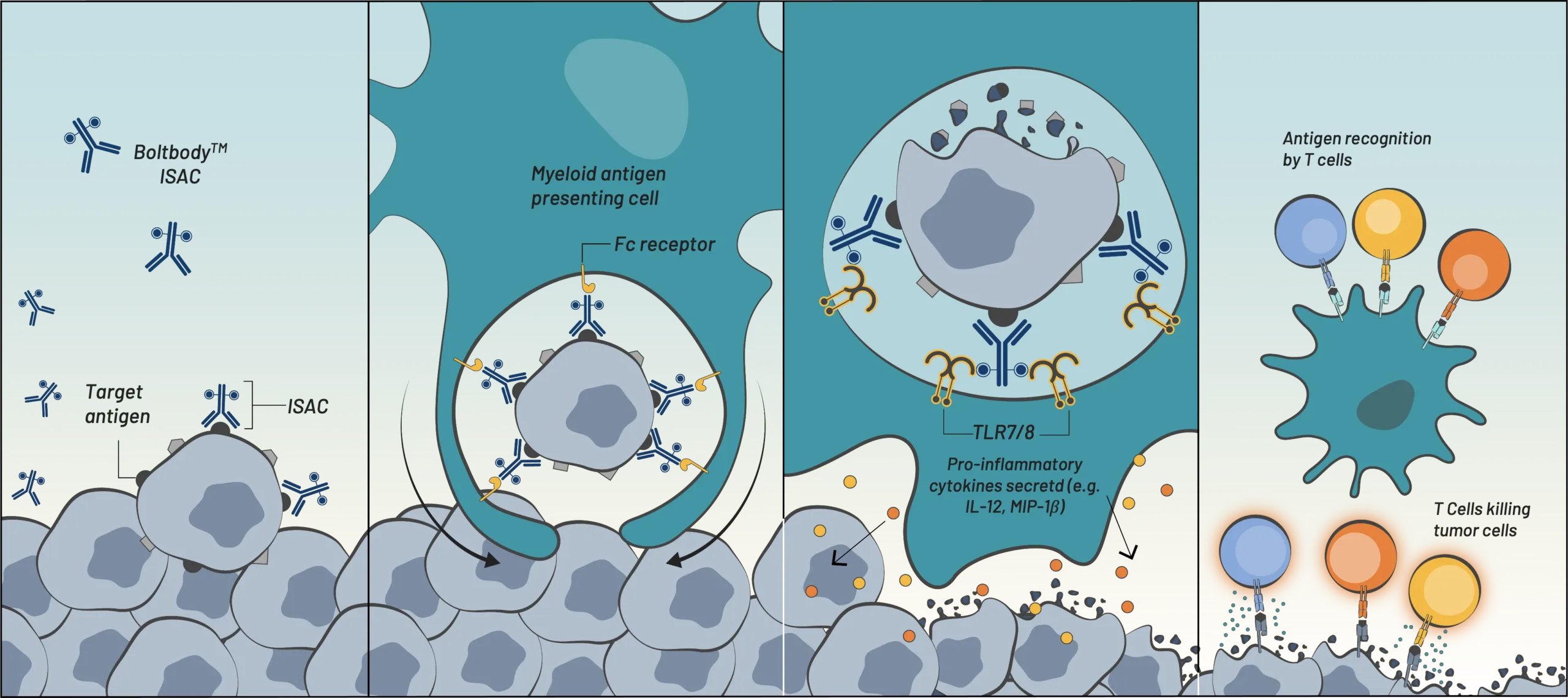
Boltbody ISAC binds
tumor antigen
Fc receptor-mediated
phagocytosis by myeloid cells
TLR7/8-mediated activation
& tumor antigen processing
Antigen presentation,
T cell recruitment,
& tumor cell killing
[Innate Immunity] ![]() [Adaptive Immunity]
[Adaptive Immunity]
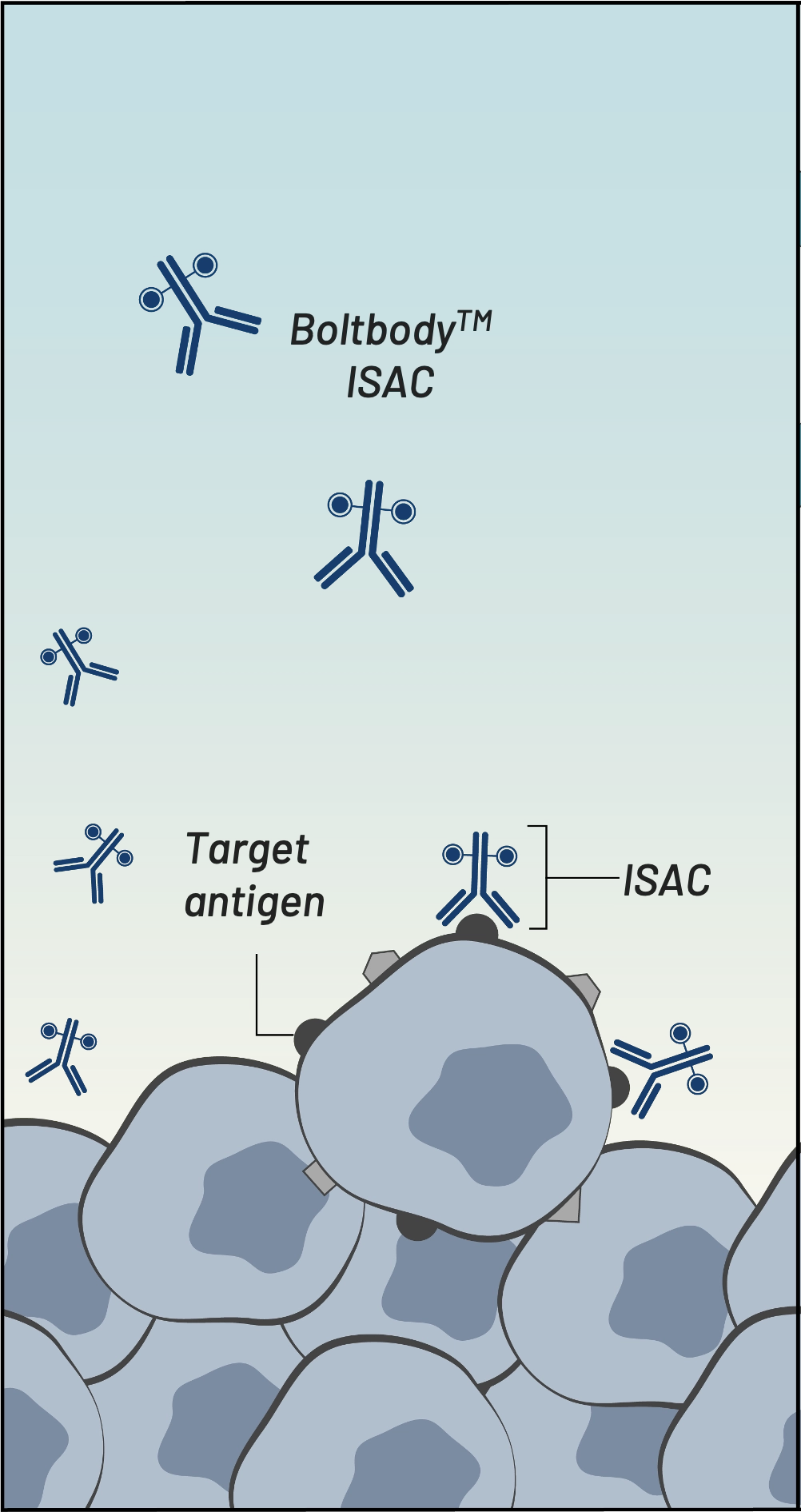
Boltbody ISAC binds tumor antigen
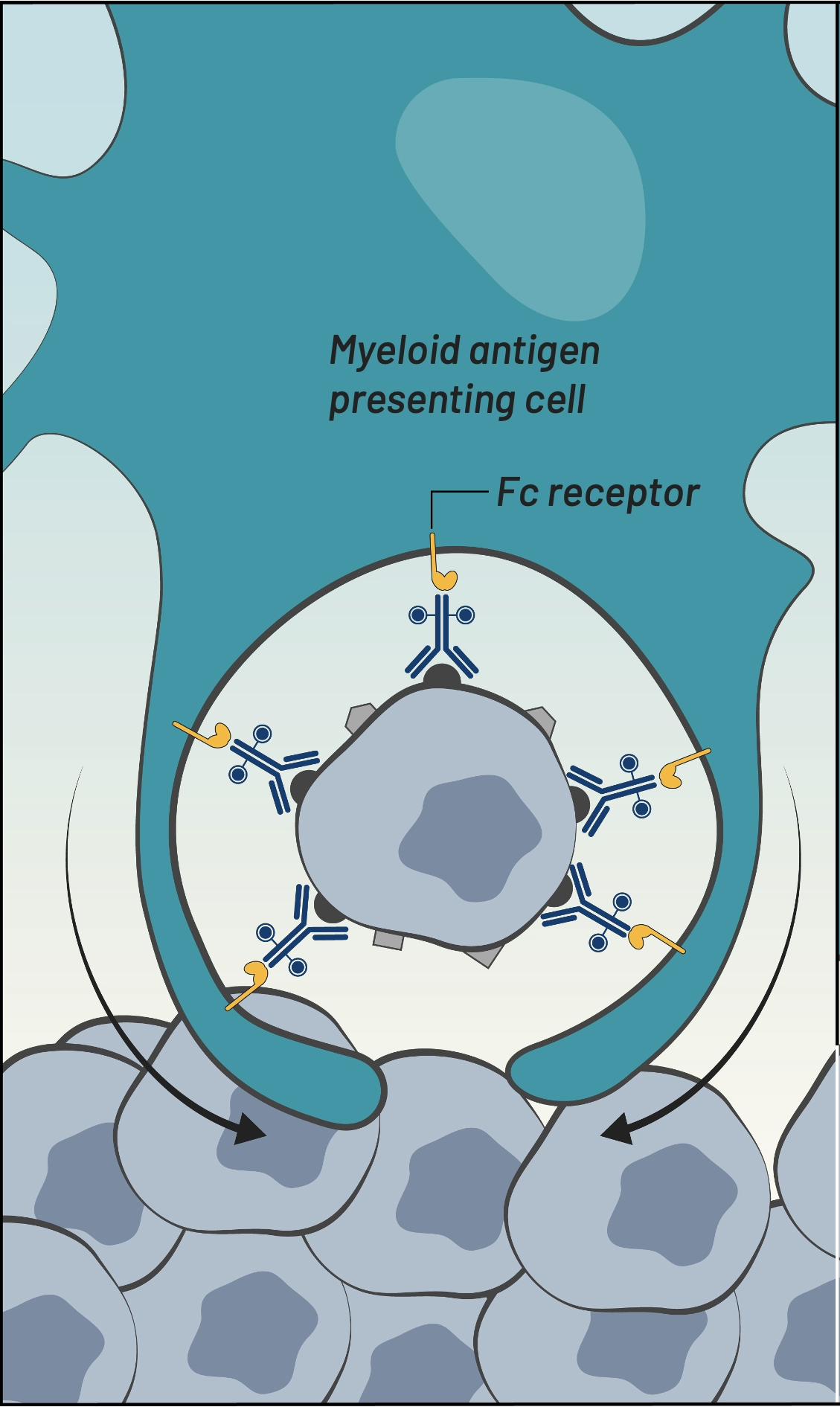
Fc receptor-mediated phagocytosis by myeloid cells
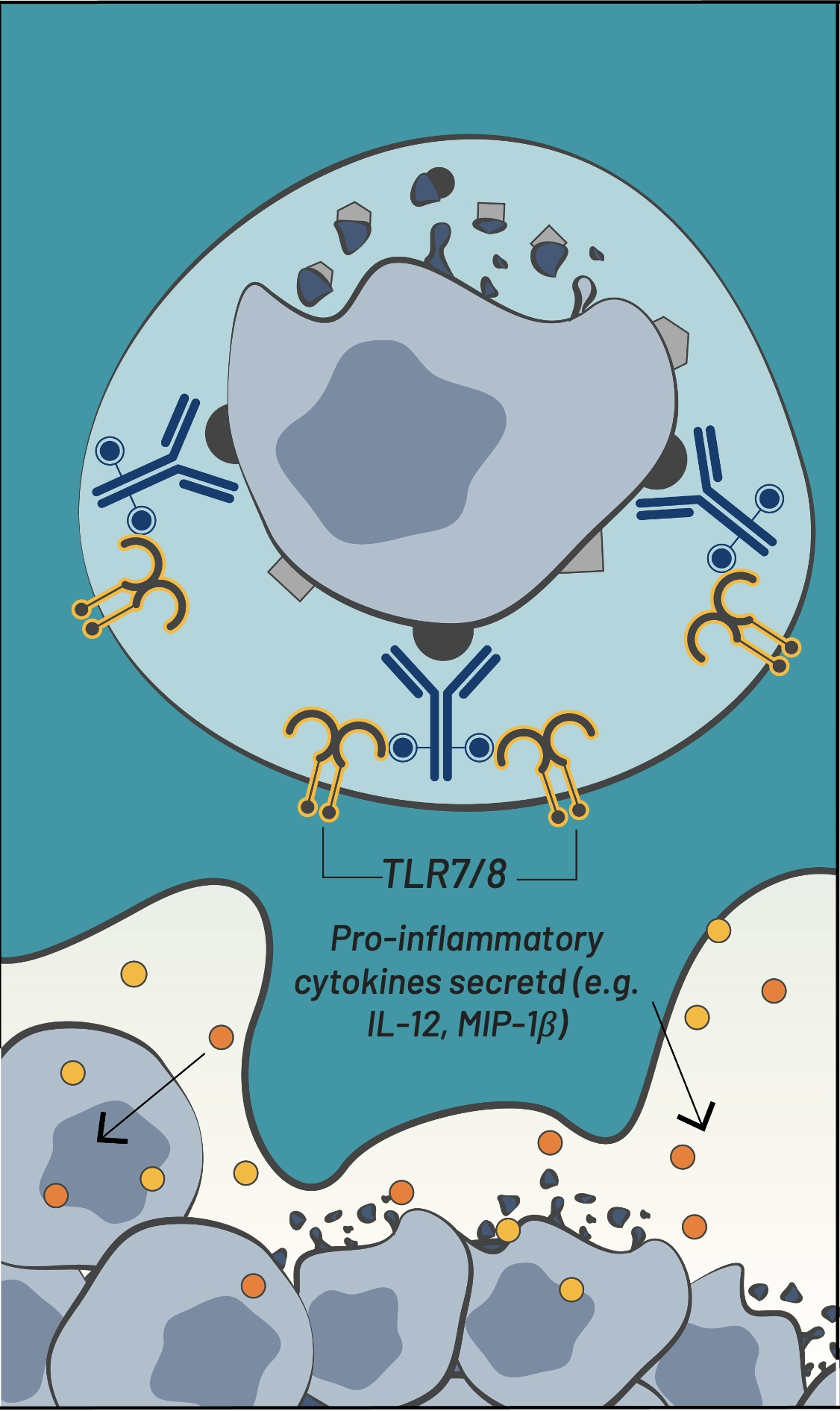
TLR7/8-mediated activation & tumor antigen processing
[Innate Immunity]

Antigen presentation, T cell recruitment, & tumor cell killing
[Adaptive Immunity]

BDC-3042
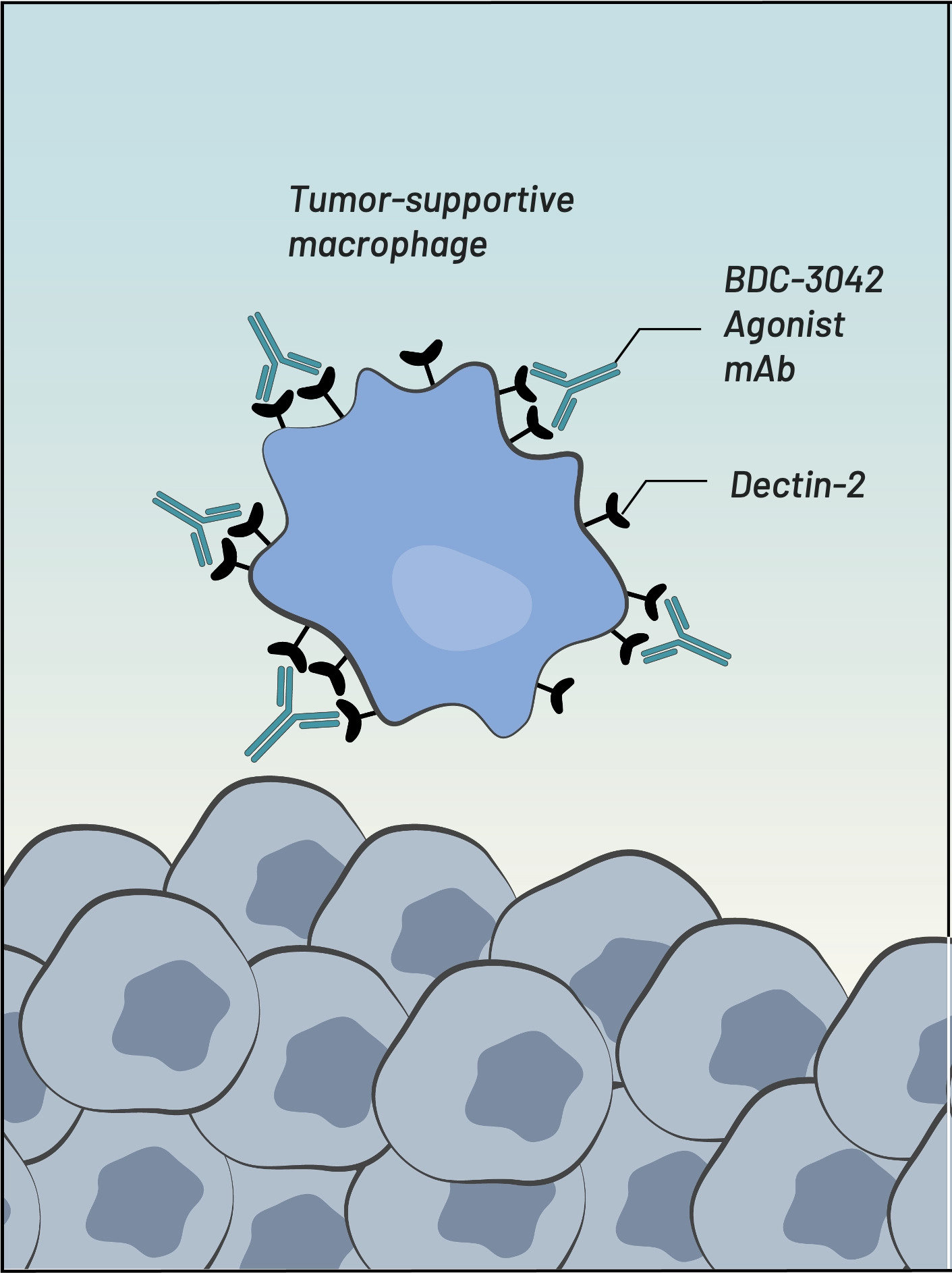
BDC-3042 is a first-in-class antibody that reprograms tumor-associated macrophages (TAMs) to attack tumor cells. BDC-3042 is a monoclonal antibody that binds to and agonizes dectin-2, a cell surface protein on tumor-supportive macrophages. The activation of these macrophages results in the production of pro-inflammatory cytokines, consistent with the characteristics of tumor-destructive macrophages. BDC-3042 has the potential to turn tumor-supportive macrophages into tumor-destructive macrophages to elicit a productive anti-tumor immune response. BDC-3042 has completed a dose escalation trial demonstrating safety and anti-tumor activity, particularly in non-small cell lung cancer at the highest dose tested.
Macrophage Repolarization
Our BDC-3042 product candidate reawakens myeloid cells to attack tumor cells. We discovered an agonist monoclonal antibody that is capable of binding to and activating a pattern-recognition receptor (known as Dectin-2) on tumor-associated macrophages (TAMs) in a broad range of solid tumors. BDC-3042 works by repolarizing TAMs from tumor-supportive to tumor-destructive.
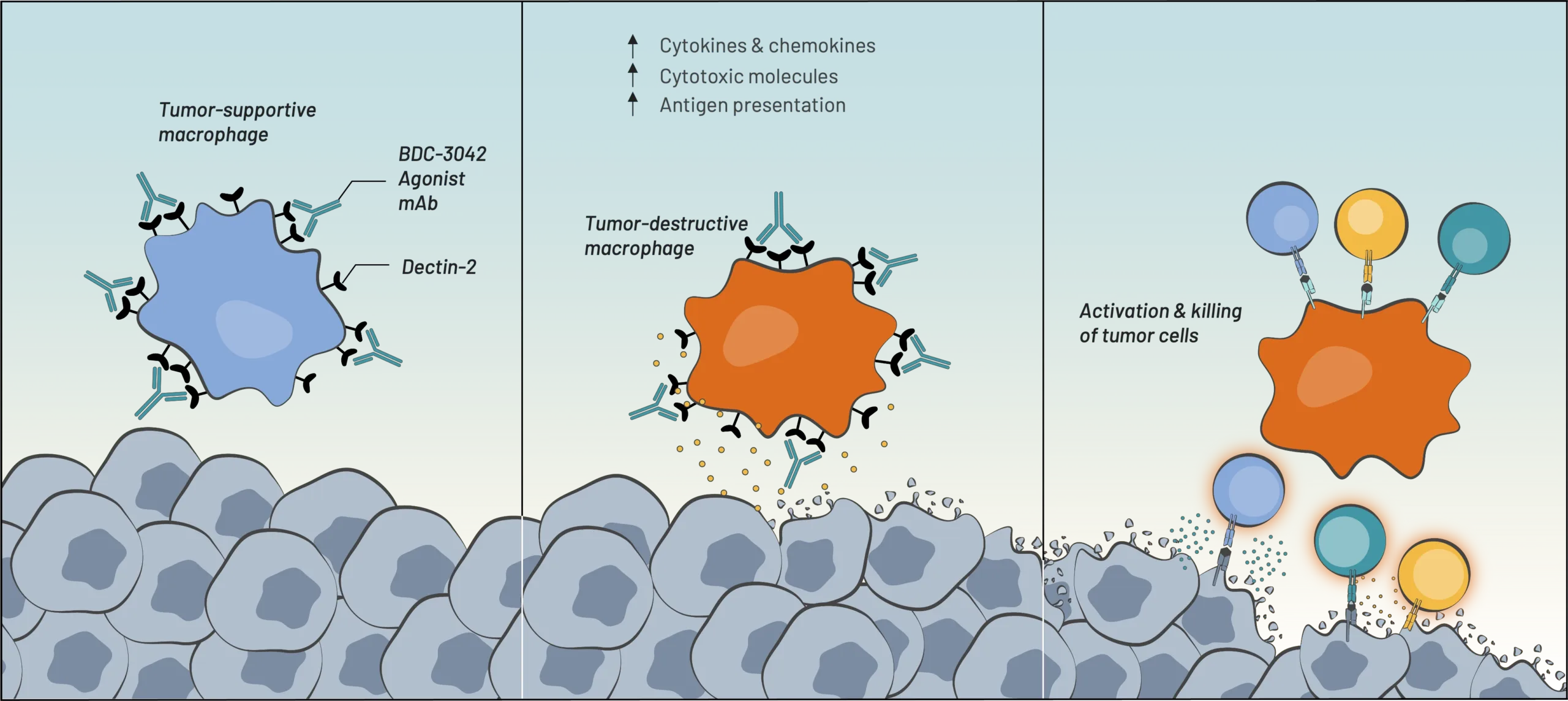
BDC-3042 binds to Dectin-2 & activates macrophages
Macrophages transform
into tumor-destructive macrophages
CD8+ T cell &
NK cell recruitment,
activation, & tumor killing
[Innate Immunity]
![]()
[Adaptive Immunity]
BDC-3042 binds to Dectin-2 & activates macrophages
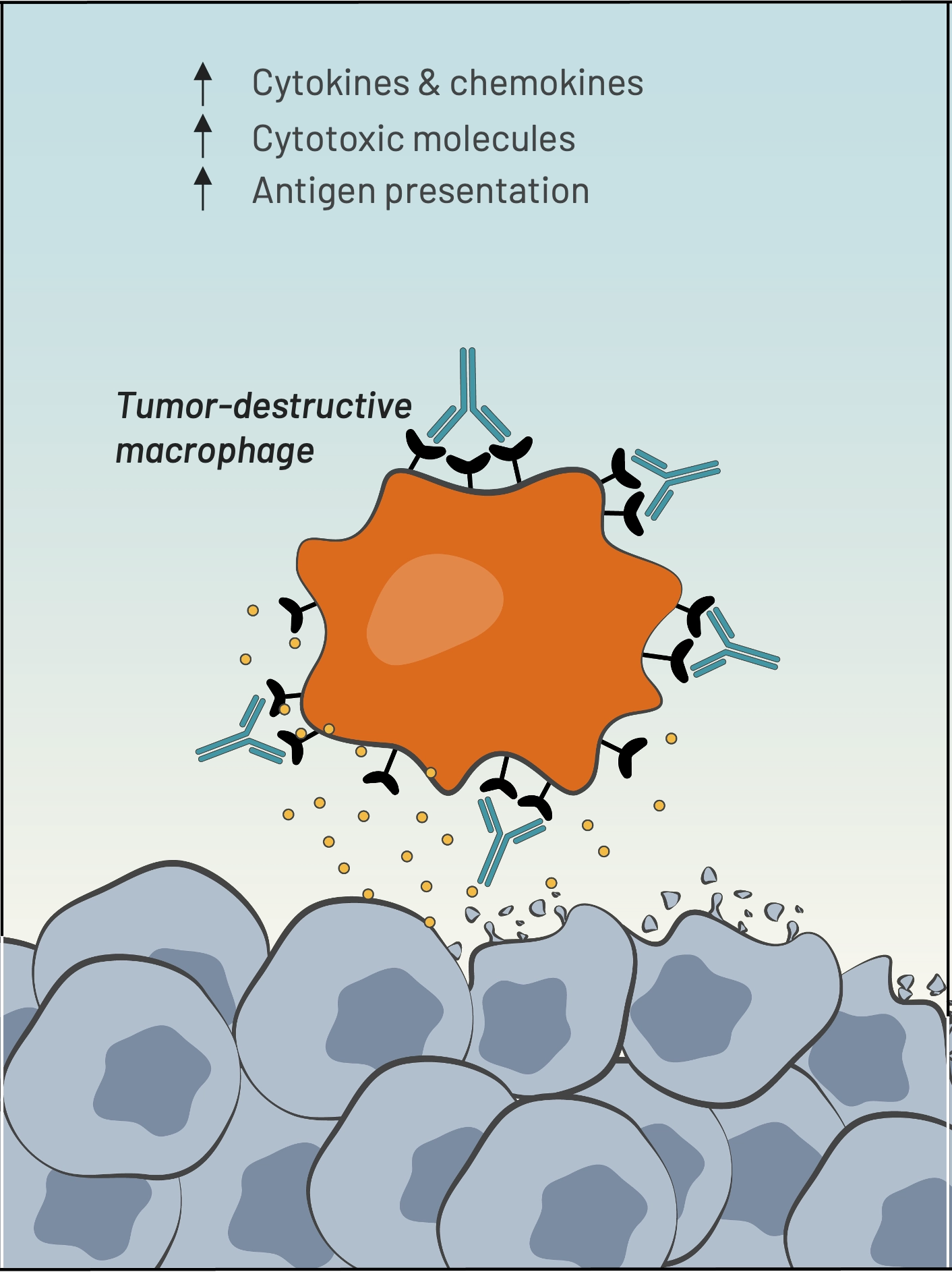
Macrophages transform into tumor-destructive macrophages
[Innate Immunity]
CD8+ T cell &
NK cell recruitment,
activation, & tumor killing
[Adaptive Immunity]

CEA ISAC
CEA refers to a specific carcinoembryonic antigen cell adhesion molecule also known as CEACAM-5 that is commonly found in gastrointestinal cancers such as colorectal cancer. Our lead CEA-targeted ISAC comprises a novel, fully human antibody with high affinity and selectivity to CEA, and not to other members of the CEACAM family, conjugated to a proprietary next-generation TLR7/8 agonist via a non-cleavable linker. This ISAC drives efficient phagocytosis of CEA-positive tumor cells by myeloid cells and stimulates production of critical immune-activating cytokines including IL-12p70, IFNg, and TNFa. In vivo, tumor growth inhibition in xenograft models demonstrates induction of myeloid cell-mediated anti-tumor activity. In a CEA transgenic syngeneic mouse model, treatment with our lead ISAC results in complete tumor regression with development of immunological memory and epitope spreading as demonstrated by failure of CEA-positive cell line and CEA-negative parental cell line to form tumors upon rechallenge. Our lead CEA ISAC was well-tolerated at the highest dose tested, 15 mg/kg, in a non-GLP toxicology study.
- SITC 2025 Presentation: Preclinical evaluation of a highly efficacious CEACAM5 (CEA) ISAC comprising a novel CEA-specific monoclonal antibody and TLR7/8 agonist
- AACR 2025 Presentation: A highly efficacious next-generation CEACAM5 (CEA)-targeted ISAC for the treatment of colorectal, pancreatic and lung tumors

PD-L1 ISAC
Our PD-L1 ISAC utilizes a novel human anti-PD-L1 antibody conjugated to a next-generation TLR7/8 agonist payload via a non-cleavable linker. This ISAC leverages a unique mechanism of action due to its ability to target both tumor and immune cells that express PD-L1. Preclinical studies show that it can directly activate PD-L1-positive antigen-presenting cells resulting in the secretion of pro-inflammatory cytokines and stimulation of powerful T cell responses. In vivo, the PD-L1 ISAC elicits complete tumor regression and immunological memory in multiple syngeneic models. Mechanistic studies have demonstrated that our PD-L1 ISAC can generate complete responses in models where PD-L1 is only expressed on the immune cells, as well as in models where PD-L1 is only expressed on the tumor cells.
- SITC 2025 Presentation: Discovery of a PD-L1-directed ISAC optimized for the activation of PD-L1-expressing myeloid cells to drive potent antitumor immune responses
- AACR 2025: PD-L1-directed ISACs target host immune cells to drive powerful antitumor immune responses in a manner distinct from conventional PD-1/PD-L1 blockade
